Clinical Trial Travel Support
Participant and Caregiver Travel, Coordinated with Compassion and Precision
Reducing burden for participants, families, site teams, and sponsors — to support retention and stronger study outcomes.
Clinical trial travel can be stressful, confusing, or financially burdensome for participants and their families. When travel becomes a barrier, visits are missed — and when visits are missed, studies slow down.
We coordinate participant and caregiver travel with compassion, clarity, and consistency.
Our support model reduces participant stress, improves access and engagement, lifts administrative workload from site staff, and protects study continuity.
We provide compliant, confidential, participant-centered travel support for sponsors, CROs, and study sites worldwide.
Key Outcomes
Clinical travel directly affects study performance. These outcomes reflect the measurable impact of supported, participant-centered travel across the full study lifecycle.
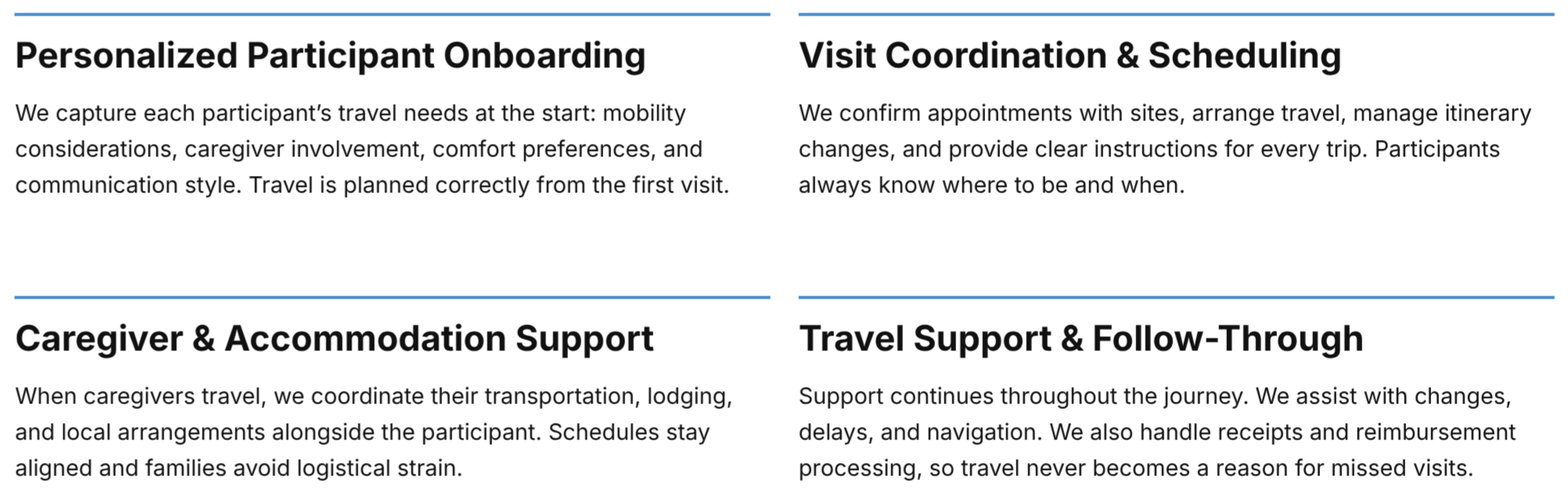
Participant Travel, Supported from Start to Finish.
We coordinate travel with accuracy and care, making each visit easier for participants and caregivers, and simpler for study teams.
No Upfront Costs, Ever.
Participants and caregivers never pay upfront or out of pocket for travel.
We arrange and pay directly for airfare, hotel (including incidentals), ground transportation, and required accommodations. There are no reimbursements to manage. This removes financial stress and barriers to continued participation.